Lithium-ion battery is a secondary battery (rechargeable battery), which mainly relies on Li + to intercalate and deintercalate between two electrodes to work. With the continuous development of downstream industries such as new energy vehicles, the production scale of lithium-ion batteries is continuously expanding. This article uses lithium cobaltate as an example to comprehensively explain the principle, formula and process of lithium-ion batteries, performance and testing of lithium batteries, production precautions and design principles.
First, the principle, formula and process of lithium-ion battery;
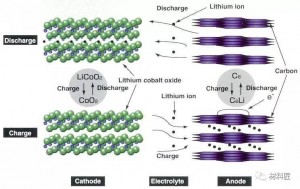
First, the working principle
1.Positive structure
LiCoO2 + conductive agent + adhesive (PVDF) + current collector (aluminum foil)
2.Negative electrode structure
Graphite + Conductive agent + Thickener (CMC) + Binder (SBR) + Current collector (copper foil)
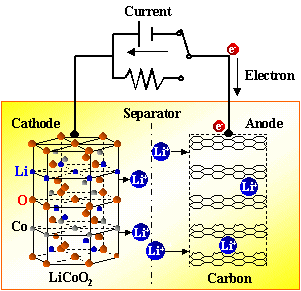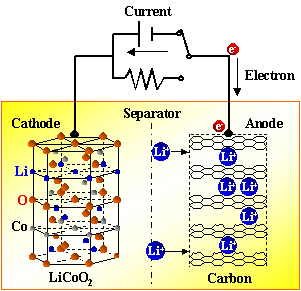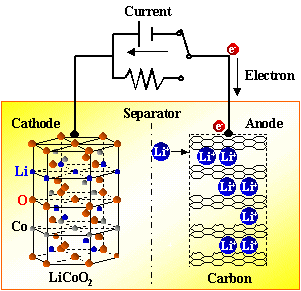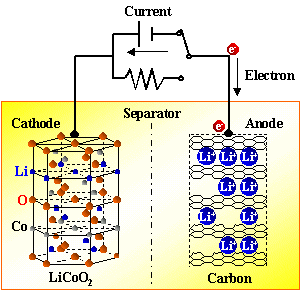
3.1 Charging process
A power source charges the battery. At this time, the electron e on the positive electrode runs from the external circuit to the negative electrode, and the positive lithium ion Li + “jumps” into the electrolyte from the positive electrode, and “crawls” through the small hole in the diaphragm. "Swimming" reaches the negative electrode and combines with the electrons that ran over long ago.
There are constant current discharge and constant resistance discharge. Constant current discharge is actually adding a variable resistor that can change with the voltage in the external circuit. The essence of constant resistance discharge is to add a resistor to the positive and negative electrodes of the battery to let electrons pass. It can be seen that as long as the electrons on the negative electrode cannot run from the negative electrode to the positive electrode, the battery will not be discharged. Both electrons and Li + move at the same time, with the same direction but different paths. During discharge, the electrons run from the negative electrode to the positive electrode through the electronic conductor, and the lithium ion Li + “jumps” into the electrolyte from the negative electrode, and “climbs” across the diaphragm The small hole, "swimming" reaches the positive electrode, and is combined with the electrons that have already come over.
3.3 Charging and discharging characteristics
The positive electrode of the cell uses LiCoO2, LiNiO2, LiMn2O2. Among them, LiCoO2 is a crystalline form with a very stable layer structure. However, when x Li ions are removed from LiCoO2, its structure may change, but whether the change depends on the x size.
It is found through research that when x> 0.5, the structure of Li1-xCoO2 appears to be extremely unstable, crystal collapse will occur, and its external appearance is the overwhelming termination of the cell. Therefore, the value of x in Li1-xCoO2 should be controlled by limiting the charging voltage during the use of the battery. Generally, the charging voltage is not greater than 4.2V and x is less than 0.5. At this time, the crystal form of Li1-xCoO2 is still stable.
The negative electrode C6 has its own characteristics. After the first formation, Li in the positive electrode LiCoO2 is charged into the negative electrode C6. When discharged, Li returns to the positive electrode LiCoO2, but after the formation, a part of Li must remain in the center of the negative electrode C6 In order to ensure the normal embedding of Li for the next charge or discharge, otherwise the overwhelming of the battery cell is very short, in order to ensure that a part of Li remains in the negative electrode C6, it is generally achieved by limiting the lower discharge voltage: safe upper limit voltage ≤ 4.2V, lower discharge The voltage is ≥2.5V.
The principle of the memory effect is crystallization, and almost no such reaction occurs in lithium batteries. However, the capacity of lithium-ion batteries will still decrease after multiple charging and discharging. The reasons are complex and diverse. It is mainly the change of the positive and negative electrode materials. From the molecular level, the hole structure that contains lithium ions on the positive and negative electrodes will gradually collapse and block. From a chemical point of view, the positive and negative electrode materials are activated and passivated, and side reactions occur. Other compounds. Physically, the positive electrode material gradually peels off, etc. In short, the number of lithium ions in the battery that can be freely moved during the charge and discharge process is eventually reduced.
Overcharge and overdischarge will cause permanent damage to the positive and negative electrodes of lithium-ion batteries. From a molecular level, it can be intuitively understood that overdischarge will cause the negative electrode carbon to release lithium ions excessively and cause its sheet structure to collapse. Overcharging will force too many lithium ions into the carbon structure of the negative electrode, and some of them can no longer be released.
Unsuitable temperatures will trigger other chemical reactions inside the lithium-ion battery to generate compounds we do not want to see, so a protective temperature-controlling separator or electrolyte additive is provided between the positive and negative electrodes of many lithium-ion batteries. When the battery temperature rises to a certain level, the membrane hole of the composite membrane is closed or the electrolyte is denatured, the internal resistance of the battery increases until it is disconnected, and the battery no longer heats up to ensure that the battery charging temperature is normal.
Second, the formula and process of lithium battery
If interested to know more details information,Please kindly find:https://www.hrl-battery.com
Post time: Nov-26-2019